
Testing PCR Clean? Efficiency
PCR Clean的科研實驗數據
INTRODUCTION
PCR Clean? is a ready-to-use solution for the removal of nucleic acids from any surface at PCR workstations and/or lab devices and equipment. The solution contains a surfactant and a non-alkaline and non-carcinogenic agent. In this study we examined the efficiency and effectiveness of PCR Clean? against different nucleic acid contaminations on various surfaces, in comparison with several other cleaning agents.
介紹
PCR Clean?是一款即用型工作液,用于去除PCR工作臺、實驗室機電產品和設備表面上留存的核酸。該工作液包含一種表面活性劑和一種非堿性非致癌性試劑。在這項研究中,我們針對不同的表面上留存的不同類型的核酸,檢查了PCR Clean?的使用效果和效率,并和其他的幾種去除劑進行了比較。
PROCEDURE
1. Removal of amplicon DNA from various surfaces
The efficiency of PCR Clean? was tested for the removal of bacterial amplicon DNA from E. coli 0104 and E. coli 0157 and compared to several other cleaning materials. Bacterial amplicon DNA was applied by pipetting 2 μl (0.05 ng) E. coli 0104 DNA on a plastic foil, glass surface, and lab work surface area (Trespa ? ), or 2 μl (0.2 ng) E. coli 0157 DNA on Plexiglas ? and aluminum-surfaces. After DNA was completely air-dried, it was removed by using a paper towel moisturized with PCR Clean?, a diluted dishwashing detergent, 70 % Ethanol, Isopropanol, water, or a dry paper towel, by wiping off the spot where DNA was pipetted with one stroke. Samples were then collected by using a moisturized swab and wiping off (swabbing) the same area. As positive control, either 0.05 ng E. coli 0104 DNA (for plastic foil, glass or Trespa ? assays), or 0.2 ng E. coli 0157 DNA (for Plexiglas ? and aluminum assays), were directly pipetted into 250 μl PCR grade water and extracted in the same manner as the other samples.
實驗過程
1、去除不同表面上的DNA擴增片段
我們用大腸桿菌O104和O157的DNA擴增片段,檢查了PCR Clean?對DNA污染的去除效率,并和其他幾種去除試劑進行了比較。我們在塑料薄膜、玻璃表面和千思板(常用于實驗工作區臺面,Trespa?品牌)滴加了2μl(0.2 ng)大腸桿菌O104 DNA,在Plexiglas?(樹脂玻璃)和鋁表面滴加了2μl(0.2ng)大腸桿菌O157 DNA。待DNA完全風干后,用蘸有PCR Clean?或一種稀釋的洗潔精、70%乙醇、異丙醇、水的紙巾擦拭去除,或者用干紙巾擦拭去除。隨后用一個干凈潮濕的拭子在該區域表面涂拭取樣。另取0.05 ng大腸桿菌O104 DNA或0.2 ng大腸桿菌O104 DNA作為陽性對照,加入250l PCR級別水溶解,其抽提方式和其他樣品一樣。
2. Removal of genomic DNA and plasmid DNA from aluminum surfaces
The efficiency of PCR Clean? was also tested for genomic and plasmid DNA removal from aluminum surfaces by pipetting 2 μl (2 × 10 6 genome copies) of E.coli genomic DNA, or 2 μl (2 × 10 6 genome copies)
2、去除鋁表面的基因組DNA和質粒DNA
在鋁表面滴加2μl(2X106拷貝數)大腸桿菌基因組DNA或質粒DNA測試。PCR Clean?與蘸水的濕紙巾或干紙巾對照。
3. Removal of RNA from a Plexiglas ? surface
5 μg (11 μl) were pipetted on each of 6 different spots of a Plexiglas ? surface.
3、去除Plexiglas?(樹脂玻璃)表面上的RNA
在Plexiglas?表面6個點分別滴加5g(11μl)RNA進行測試。取樣后的RNA作逆轉錄和qPCR。
4. DNA extraction and qPCR amplification
DNA extraction from the swabs was carried out using our SwabUp? Lab Monitoring kit. DNA was extracted from all
collected samples and positive controls in the same manner. All DNA extracts were subsequently subjected to qPCR.
4、DNA抽提和qPCR擴增
拭子上的DNA采用SwabUp? Lab Monitoring試劑盒進行抽提。所有收集的樣本DNA和陽性對照抽提的方式相同。隨后進行qPCR。
RESULTS
1. Removal of amplicon DNA from various surfaces
Results showed a bigger depletion of amplicon DNA after removal with PCR Clean? from the various surfaces tested, in comparison to other cleaning agents and in reference to the positive control (Table 1). For all surfaces tested, PCR Clean? showed the best effectiveness for the removal of amplicon DNA, which could be observed in Graph 1, where Ct -values for PCR Clean? were higher in comparison to other cleaning agents and in reference to the positive control, regardless of the surface material (Graph 1, A-E), indicating a higher decrease in DNA amount.
檢驗結果
1、去除不同表面的DNA擴增片段
結果顯示,和其他幾種清除劑相比,PCR Clean?的對擴增的DNA片段去除的更多(見表1),效果**(見圖1),Ct值更高。
表1:與其他清除劑相比,使用PCR Clean?后,qPCR測定的細菌DNA擴增片段的Ct值
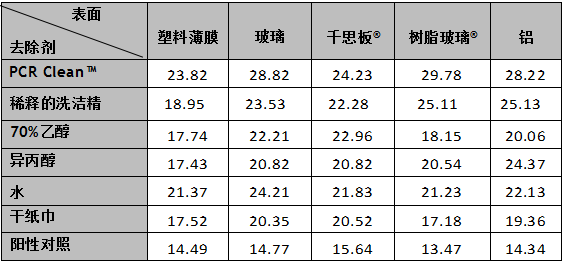
圖1:采用不同的清除劑,對 A.樹脂玻璃?、B.鋁、C.千思板?、D.玻璃和E.塑料薄膜上的大腸桿菌DNA擴增片段去除后,qPCR的擴增曲線
2. Removal of genomic DNA and plasmid DNA from aluminum surfaces
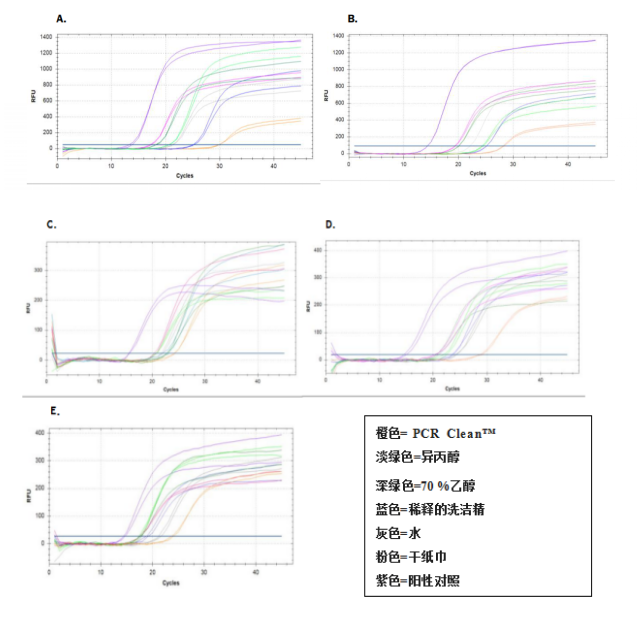
Results showed a bigger depletion of genomic DNA and plasmid DNA after removal with PCR Clean? from an aluminum surface
2、去除鋁表面的基因組DNA和質粒DNA
結果顯示,與水和干紙巾相比,含PCR Clean?的濕巾對基因組DNA和質粒DNA有更多的去除(見表2)。Ct值(圖2,A,B)更高,提示DNA量有大幅下降。
表2:與水和干紙巾相比,使用PCR Clean?后,qPCR測定的鋁表面基因組DNA或質粒DNA的Ct值
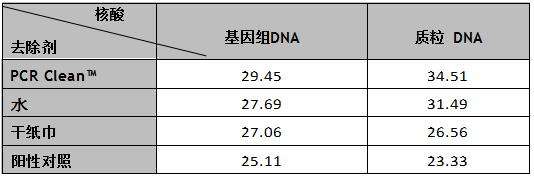
圖2:采用PCR Clean?、水或干紙巾去除鋁表面的大腸桿菌基因組DNA或大腸桿菌質粒DNA后的qPCR擴增曲線
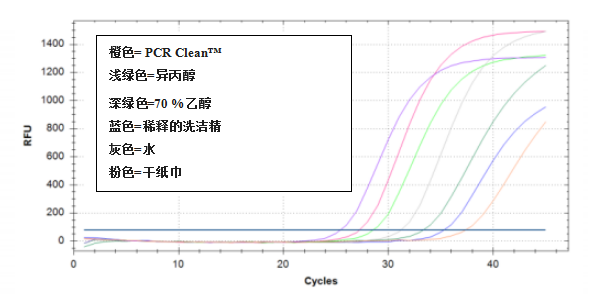
3. Removal of RNA from a Plexiglas ? surface
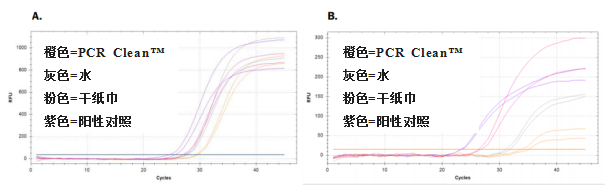
Results showed a bigger depletion of RNA after removal with PCR Clean? from a Plexiglas ? surface
3、去除Plexiglas?(樹脂玻璃)表面的RNA
和其他幾種清除劑相比,PCR Clean?對Plexiglas?表面的RNA去除最多(見表3),Ct值也最高(見圖3)
表3:和其他幾種清除劑相比,PCR Clean?去除樹脂玻璃表面的RNA后,RNA逆轉錄合成cDNA, 再qPCR測定Ct值
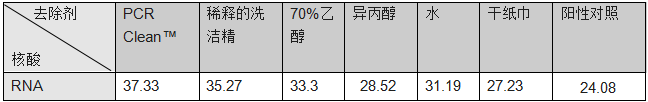
圖3:使用不同的清除劑,從樹脂玻璃?中去除RNA后,RNA再逆轉錄并進行qPCR擴增
CONCLUSIONS
The results of our study showed that PCR Clean? is highly effective against amplicon, plasmid, and genomic DNA, and RNA contaminations from all surfaces tested, even within seconds after use. Depletion results also demonstrate superiority in effectiveness and efficiency of PCR Clean? compared to any of the other common cleaning agents usually available at molecular biology laboratories, e.g. ethanol, isopropanol or dishwashing detergent. Therefore, the regular use of PCR Clean?, both before and after PCR analysis is fast, easy and ideal to maintain a clean work area, which can be critical in molecular biology laboratories and PCR workstations, and thereby saves time and expenses.
結論
我們研究的結果顯示,PCR Clean?對來自所有檢測表面的DNA擴增片段,質粒、基因組DNA和RNA污染的去除,是極為有效的,而且起效很快。去除結果還顯示,相比于其他常見的DNA清除劑,比如分子生物學實驗室常用的乙醇、異丙醇或洗潔精,PCR Clean?的去除效果和效率都具有明顯的優勢。因此,在PCR反應前后,經常使用PCR Clean?,會非常便捷,可理想地保持干凈的工作區域,而這對于分子生物學實驗室和PCR工作臺都是非常重要的,因為它可節省時間和支出。
 細胞培養進口血清進口胎牛血清進口新生牛血清進口豬血清馬血清
細胞培養進口血清進口胎牛血清進口新生牛血清進口豬血清馬血清 支原體檢測盒及標準品常規PCR檢測試劑盒熒光定量PCR檢測(qPCR法)支原體DNA提取靈敏度標準品(方法驗證用)特異性標準品(方法驗證用)PCR定量標準品(可用于方法驗證)
支原體檢測盒及標準品常規PCR檢測試劑盒熒光定量PCR檢測(qPCR法)支原體DNA提取靈敏度標準品(方法驗證用)特異性標準品(方法驗證用)PCR定量標準品(可用于方法驗證) 支原體祛除試劑細胞中支原體祛除環境支原體祛除水槽支原體祛除
支原體祛除試劑細胞中支原體祛除環境支原體祛除水槽支原體祛除 干細胞培養基
干細胞培養基 DNA/RNA污染祛除DNA/RNA污染祛除試劑DNA污染監測
DNA/RNA污染祛除DNA/RNA污染祛除試劑DNA污染監測 RNA病毒研究試劑RNA病毒檢測試劑盒病毒RNA提取
RNA病毒研究試劑RNA病毒檢測試劑盒病毒RNA提取 PCR儀器及配套產品DNA污染監測祛除PCR/qPCR儀性能檢查PCR試劑PCR試劑盒PCR預混液(凍干粉)熱啟動聚合酶MB Taq DNA
PCR儀器及配套產品DNA污染監測祛除PCR/qPCR儀性能檢查PCR試劑PCR試劑盒PCR預混液(凍干粉)熱啟動聚合酶MB Taq DNA 微生物PCR檢測食品檢測類產品食品微生物檢測細菌PCR檢測
微生物PCR檢測食品檢測類產品食品微生物檢測細菌PCR檢測 細胞培養進口血清
細胞培養進口血清 支原體祛除試劑
支原體祛除試劑 干細胞培養基
干細胞培養基









