細(xì)胞培養(yǎng)進(jìn)口血清進(jìn)口胎牛血清進(jìn)口新生牛血清進(jìn)口豬血清馬血清
細(xì)胞培養(yǎng)進(jìn)口血清進(jìn)口胎牛血清進(jìn)口新生牛血清進(jìn)口豬血清馬血清 支原體檢測(cè)盒及標(biāo)準(zhǔn)品常規(guī)PCR檢測(cè)試劑盒熒光定量PCR檢測(cè)(qPCR法)支原體DNA提取靈敏度標(biāo)準(zhǔn)品(方法驗(yàn)證用)特異性標(biāo)準(zhǔn)品(方法驗(yàn)證用)PCR定量標(biāo)準(zhǔn)品(可用于方法驗(yàn)證)
支原體檢測(cè)盒及標(biāo)準(zhǔn)品常規(guī)PCR檢測(cè)試劑盒熒光定量PCR檢測(cè)(qPCR法)支原體DNA提取靈敏度標(biāo)準(zhǔn)品(方法驗(yàn)證用)特異性標(biāo)準(zhǔn)品(方法驗(yàn)證用)PCR定量標(biāo)準(zhǔn)品(可用于方法驗(yàn)證) 支原體祛除試劑細(xì)胞中支原體祛除環(huán)境支原體祛除水槽支原體祛除
支原體祛除試劑細(xì)胞中支原體祛除環(huán)境支原體祛除水槽支原體祛除 干細(xì)胞培養(yǎng)基
干細(xì)胞培養(yǎng)基 DNA/RNA污染祛除DNA/RNA污染祛除試劑DNA污染監(jiān)測(cè)
DNA/RNA污染祛除DNA/RNA污染祛除試劑DNA污染監(jiān)測(cè) RNA病毒研究試劑RNA病毒檢測(cè)試劑盒病毒RNA提取
RNA病毒研究試劑RNA病毒檢測(cè)試劑盒病毒RNA提取 PCR儀器及配套產(chǎn)品DNA污染監(jiān)測(cè)祛除PCR/qPCR儀性能檢查PCR試劑PCR試劑盒PCR預(yù)混液(凍干粉)熱啟動(dòng)聚合酶MB Taq DNA
PCR儀器及配套產(chǎn)品DNA污染監(jiān)測(cè)祛除PCR/qPCR儀性能檢查PCR試劑PCR試劑盒PCR預(yù)混液(凍干粉)熱啟動(dòng)聚合酶MB Taq DNA 微生物PCR檢測(cè)食品檢測(cè)類產(chǎn)品食品微生物檢測(cè)細(xì)菌PCR檢測(cè)
微生物PCR檢測(cè)食品檢測(cè)類產(chǎn)品食品微生物檢測(cè)細(xì)菌PCR檢測(cè)
- 細(xì)胞培養(yǎng)進(jìn)口血清進(jìn)口胎牛血清進(jìn)口新生牛血清進(jìn)口豬血清馬血清
- 支原體檢測(cè)盒及標(biāo)準(zhǔn)品常規(guī)PCR檢測(cè)試劑盒熒光定量PCR檢測(cè)(qPCR法)支原體DNA提取靈敏度標(biāo)準(zhǔn)品(方法驗(yàn)證用)特異性標(biāo)準(zhǔn)品(方法驗(yàn)證用)PCR定量標(biāo)準(zhǔn)品(可用于方法驗(yàn)證)
- 支原體祛除試劑細(xì)胞中支原體祛除環(huán)境支原體祛除水槽支原體祛除
- 干細(xì)胞培養(yǎng)基
- DNA/RNA污染祛除DNA/RNA污染祛除試劑DNA污染監(jiān)測(cè)
- RNA病毒研究試劑RNA病毒檢測(cè)試劑盒病毒RNA提取
- PCR儀器及配套產(chǎn)品DNA污染監(jiān)測(cè)祛除PCR/qPCR儀性能檢查PCR試劑PCR試劑盒PCR預(yù)混液(凍干粉)熱啟動(dòng)聚合酶MB Taq DNA
- 微生物PCR檢測(cè)食品檢測(cè)類產(chǎn)品食品微生物檢測(cè)細(xì)菌PCR檢測(cè)
|
|
污染細(xì)胞的支原體從哪里來(lái)2016-09-23 13:40
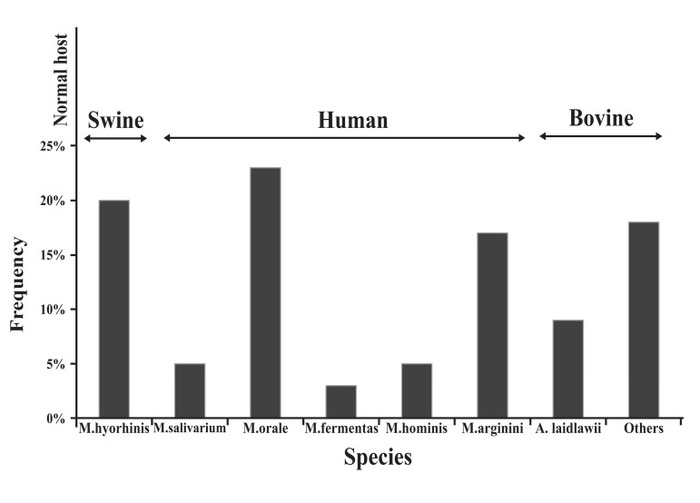
締一生物導(dǎo)讀: 在培養(yǎng)細(xì)胞時(shí),人們常常關(guān)注細(xì)菌和真菌的污染,認(rèn)為它們是細(xì)胞培養(yǎng)的主要干擾因素。但其實(shí),更嚴(yán)重的是支原體 的感染,它的發(fā)生率非常高,而且不易被發(fā)現(xiàn)。不僅普通光鏡下無(wú)法發(fā)現(xiàn)支原體,而且培養(yǎng)基也不容易變色。但支原體對(duì)細(xì)胞的各種干擾卻是不容忽視的。它不僅導(dǎo)致細(xì)胞狀態(tài)不佳,生長(zhǎng)速度慢,而且會(huì)使細(xì)胞內(nèi)DNA、RNA及蛋白表達(dá)發(fā)生改變,嚴(yán)重影響實(shí)驗(yàn)結(jié)果。因此,在預(yù)防和治療支原體污染之前,有必要去了解細(xì)胞被支原體污染的可能途徑。 支原體污染細(xì)胞的途徑(點(diǎn)擊這里,詳細(xì)了解支原體污染防治類試劑) 支原體可以通過(guò)頂部的細(xì)胞器特異性和宿主細(xì)胞結(jié)合。這些頂部的細(xì)胞器含有高濃度的粘附蛋白,可粘附到真核細(xì)胞并穿入到細(xì)胞內(nèi)部。支原體缺乏細(xì)胞壁,它們的胞膜可和宿主的細(xì)胞膜融合并交換其胞膜和胞漿成分。 支原體污染的頻率和污染來(lái)源 細(xì)胞培養(yǎng)中有幾種支原體污染與人、牛和豬有關(guān)。實(shí)驗(yàn)室的工作人員是口腔支原體、發(fā)酵支原體和人型支原體的主要來(lái)源。這三種支原體占細(xì)胞培養(yǎng)中支原體污染的一半以上,它們主要存在于人的口咽部。精氨酸支原體和無(wú)膽甾原體是另兩類細(xì)胞培養(yǎng)中的支原體,它們來(lái)自胎牛血清和新生牛血清。胰酶如果是豬來(lái)源的,那么也可能存在豬鼻支原體。圖1顯示了支原體的常見(jiàn)宿主以及污染細(xì)胞的發(fā)生率。
圖1:在細(xì)胞培養(yǎng)中出現(xiàn)的不同種類的支原體頻率 支原體在實(shí)驗(yàn)室內(nèi)的擴(kuò)散來(lái)源 McGarrity設(shè)計(jì)了一個(gè)模型,來(lái)發(fā)現(xiàn)支原體是如何在超凈臺(tái)里的細(xì)胞傳代過(guò)程中發(fā)生傳播的。他先故意用支原體感染細(xì)胞。在超凈臺(tái)中,用胰蛋白酶消化該污染的細(xì)胞后發(fā)現(xiàn),活的支原體可從細(xì)胞培養(yǎng)瓶外的細(xì)胞計(jì)數(shù)板、移液器、廢棄盤中被技術(shù)員分離出來(lái)。即使過(guò)了四至六天,活的支原體仍然可以存在于超凈臺(tái)表面,可被成功恢復(fù)。在超凈臺(tái)里,傳代完被支原體污染的細(xì)胞后,再傳代干凈的不含支原體的細(xì)胞,結(jié)果仍然在6周后被檢測(cè)出支原體陽(yáng)性。這些結(jié)果表明,支原體的傳播是多么的快速和容易!它也警告我們,要**限度的避免支原體的污染,因?yàn)橐黄考?xì)胞發(fā)生污染,就會(huì)帶來(lái)支原體傳播的可能。 未真正消毒的耗材、培養(yǎng)基和溶液 不恰當(dāng)?shù)南緦?dǎo)致污染的另一個(gè)原因。高壓鍋或干熱烤箱中堆積太多的物品造成加熱不均,使一小部分耗材未得到消毒。滅菌循環(huán)時(shí)間過(guò)短是另一個(gè)消毒上犯的錯(cuò)誤,特別是對(duì)于超過(guò)500ml的液體,或者是包含固體成分的溶液,或膠體物質(zhì)如瓊脂、淀粉等。為了實(shí)現(xiàn)無(wú)菌,滅菌材料的尺寸、質(zhì)量、性質(zhì)和體積必須始終考慮。 在無(wú)菌和無(wú)昆蟲(chóng)區(qū)域存放消毒好的物品,使防止再次污染的前提。良好的無(wú)菌操作也是至關(guān)重要的。 實(shí)驗(yàn)室人員 在因人導(dǎo)致的細(xì)胞支原體污染中,口腔支原體發(fā)生率最高,它主要存在人的口咽部。發(fā)酵支原體和唾液支原體也在污染的細(xì)胞培養(yǎng)液中被檢測(cè)出來(lái),不過(guò)發(fā)生率更低。 被支原體污染的細(xì)胞 實(shí)驗(yàn)室內(nèi)細(xì)胞支原體的相互傳染,有必要對(duì)于從外源獲得的新的細(xì)胞系進(jìn)行支原體檢測(cè)。一瓶細(xì)胞中的單個(gè)支原體的存在足以威脅到其他培養(yǎng)的細(xì)胞。支原體的污染能通過(guò)細(xì)胞操作產(chǎn)生的氣溶膠或液滴傳播。所以,應(yīng)一次只操作一種細(xì)胞,為每種細(xì)胞系配置單獨(dú)的培養(yǎng)基和試劑,這能避免支原體的污染。 細(xì)胞培養(yǎng)的正確操作和對(duì)新培養(yǎng)的細(xì)胞定期檢查,可以減少支原體污染的機(jī)會(huì)。 威正翔禹生物原裝進(jìn)口Ausbian特級(jí)胎牛血清,內(nèi)毒素低,品質(zhì)高,為您預(yù)留庫(kù)存,同批號(hào)跟蹤實(shí)驗(yàn)數(shù)據(jù)更穩(wěn)定,血清全程無(wú)凍融,讓細(xì)胞表現(xiàn)更出色!另外還有各種支原體檢測(cè)祛除試劑、細(xì)胞培養(yǎng)基、微生物培養(yǎng)基等,威正翔禹生物,為您科研室物料提供有力保障! 英文原文: Ways in which cells are contaminated by mycoplasma Mycoplasmas can bind to their host cells using special tip organelles. These tip organelles have a high concentration of adhesins, to attach to eukaryotic cells and penetrate the host cell. The lack of a stiff wall in mycoplasma may help it to fuse with the membrane of the host cell and exchange its membrane and cytoplasmic components. Frequency and sources of mycoplasma species There are a number of different sources for mycoplasma contamination in cell cultures associated with human, bovine and swine species. Personnel in the laboratories are the main sources of M. orale, M. fermentans, and M. hominis. These species of mycoplasmas account for more than half of all mycoplasma infections in cell cultures and physiologically are found in the human oropharyngeal tract . M. arginini and A. laidlawii are two other mycoplasmas contaminating cell cultures and originate in fetal bovine serum (FBS) or newborn bovine serum (NBS)。 Trypsin solutions provided by swines are a major source of M. hyorhinis. Figure 1 is a diagram showing the normal host and frequency of different species of mycoplasma occurring in cell culture. Different sources for the spreading of mycoplasma in the laboratory McGarrity designed a model to find out how mycoplasmas spread in a laminar flow hood during a routine subculturing procedure. He intentionally infected a cell culture with mycoplasma. After trypsinization of the infected culture in a laminar flow hood, live mycoplasmas were isolated by the technician, outside of the flask, a hemocytometer, the pipettor, and outside of the pipette discard pan. Live mycoplasma could be successfully recovered from the surface of the laminar flow hood even four to six days later! A clean culture, that was subcultured once a week in the same hood following the work with the contaminated cells, tested positive for mycoplasma after only 6 weeks. These results show how quickly and easily mycoplasma can spread and also warn us against the possibility of contamination of most if not all of the other cultures after the entry of a single mycoplasma infected culture into the laboratory. Nonsterile supplies, media and solutions Improper sterilization is a major source of biological contaminants. Packing too much into an autoclave or dry heat oven will cause uneven heating, resulting in pockets of nonsterile supplies. Using too short a sterilization cycle, especially for autoclaving volumes of liquids greater than 500 ml per vessel or solutions containing solids or viscous materials such as agar or starches are other mistakes resulting in incorrect sterilization. To accomplish sterility, the size, mass, nature and volume of the materials for sterilization have to always be considered . Storing sterilized supplies and solutions in a dust- and insect-free area is an obligation to prevent recontamination. Good aseptic technique is also crucial Laboratory personnel Laboratory personnel are considered a major source of mycoplasma contamination . Table 1 shows potential sources of cell culture contamination. M. orale, a species commonly found colonizing the human oral cavity and oropharynx, has been the leading contaminant in study after study. Two other human mycoplasma species, M. fermentans and M. salivarium, are also detected in contaminated cultures but at a much lower rate. Table 2 shows major mycoplasma species found in cell culture and also some of the research results reporting the percentage of contamination with different types of mycoplasma in previous years . Table 1: Potential sources of cell culture contamination. Other mycoplasma contaminated cell cultures A mycoplasma-infected cell culture is a major source of mycoplasma contamination of other cell cultures in the lab. To avoid mycoplasma contamination in cell cultures, it is recommended to test the new cell lines which are obtained from an outside source. A single mycoplasma contaminated cell culture is enough to endanger other cell cultures in the lab. The contamination can spread by means of aerosols and particulates generated during the handling of the mycoplasma infected cell culture. So, working with only one cell culture at a time and preparing separate media and reagents for each individual cell line can avert mycoplasma contamination . A good cell culture practice and regular testing of all new cell cultures can decrease the risk of mycoplasma contamination . 文獻(xiàn)出處:N Laleh,F(xiàn) Parvaneh. Prevention and Detection of Mycoplasma Contamination in Cell Culture. Cell Journal, 2012, 13(4): 203 版權(quán)聲明:版權(quán)歸原作者所有,如有版權(quán)問(wèn)題,請(qǐng)與我們聯(lián)系。
|
 細(xì)胞培養(yǎng)進(jìn)口血清
細(xì)胞培養(yǎng)進(jìn)口血清 支原體祛除試劑
支原體祛除試劑 干細(xì)胞培養(yǎng)基
干細(xì)胞培養(yǎng)基